CXCL10: a promising marker for immunotherapy response in metastatic melanoma
Immunotherapy has emerged as a major new type of treatment for cancer. This form of therapy is a way of harnessing a patient’s own immune system to recognize and destroy tumor cells. One of the most widely utilized type of immunotherapy drug targets an immune inhibitory pathway called PD-1. Drugs blocking PD-1 have been FDA approved for 15 distinct cancer entities. Despite this important success, many patients do not respond to anti-PD-1 therapy. The laboratory of Thomas Gajewski, MD, PhD, has been focusing on identifying the mechanisms involved when anti-PD-1 works, in order to develop new therapies that can turn non-responders into responders.
Gajewski is a clinician-scientist and the AbbVie Foundation Professor of Cancer Immunotherapy. His lab studies how a type of immune cells, called T cells, can fight melanoma and other cancers. The Gajewski laboratory previously established that, in order for T cells to fight cancer, the T cells need to migrate into the tumor microenvironment. A major factor driving the attraction of T cells into tumor sites was found to be a chemokine called CXCL10. This chemokine binds a receptor on activated T cells called CXCR3, which promotes directional trafficking of T cells into the tumor.
In a recent study published in the Journal of Immunotherapy of Cancer, an MD fellow in the lab, Robin Reschke, built upon this previous work from mouse models to determine whether a similar mechanism might be involved in human melanoma patients treated with anti-PD-1.
“One major goal is understanding how T cells infiltrate tumors,” said Reschke. “If we can confirm this mechanism, then perhaps we can develop new therapies that induce T-cell recruitment to cancer sites to expand immunotherapy effectiveness.”
For the recently published study, Reschke and colleagues used biopsies from patients with advanced metastatic melanoma. The biopsies were taken before the patients went on to receive immunotherapy. They found that CXCL10 mRNA transcripts were highly variable between patients. Importantly, high CXCL10 expression was predictive of subsequent clinical response to anti-PD-1 treatment.
“These data argue that the ability of the tumor to recruit T cells is critical for immunotherapy effectiveness,” said Gajewski. “This work should motivate the development of new therapeutic interventions that support T-cell migration into tumors that fail to do that on their own.”
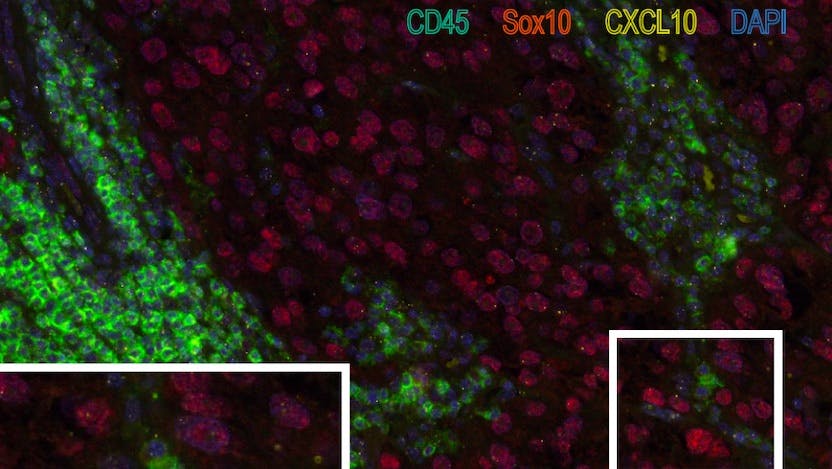
An additional goal of this study was to identify the cellular sources of CXCL10 by capitalizing on a commercialized technology known as RNAscope. This technology allows someone to see by fluorescence where (i.e., in which cell types) specific RNA molecules are produced. They found that the mRNA needed for making CXCL10 protein was most abundant in immune cells (mostly dendritic cells and macrophages) but also in the tumor cells (see image).
Reschke remarked that technologies like RNAscope are valuable because there is the possibility to make use of biobank tissues that have been carefully preserved using standard methods. He believes there is a future where pathologists could adopt these methods for diagnosis.
Reschke said, “Since checkpoint blockade immunotherapy works best in a T cell-inflamed tumor microenvironment, I think there will be a huge opportunity to make use of this crosstalk between the tumor microenvironment and T cells, orchestrated via chemokines, to markedly improve the efficacy of anti-PD-1.”

Thomas F. Gajewski, MD, PhD
Thomas Gajewski, MD, PhD, investigates and develops new treatments for patients with melanoma. He also leads development of immune-based therapies for other cancers, using new laboratory data on how the immune system is regulated to develop novel clinical trials.
Read Dr. Gajewski's physician profile
Cancer Immunotherapy
Immunotherapy refers to a medical treatment that turns the power of the immune system against disease. Cancer immunotherapy acts on the cells of the immune system to seek out, recognize and attack cancer.
Learn about immunotherapy options to treat cancer