The immunotherapy revolution
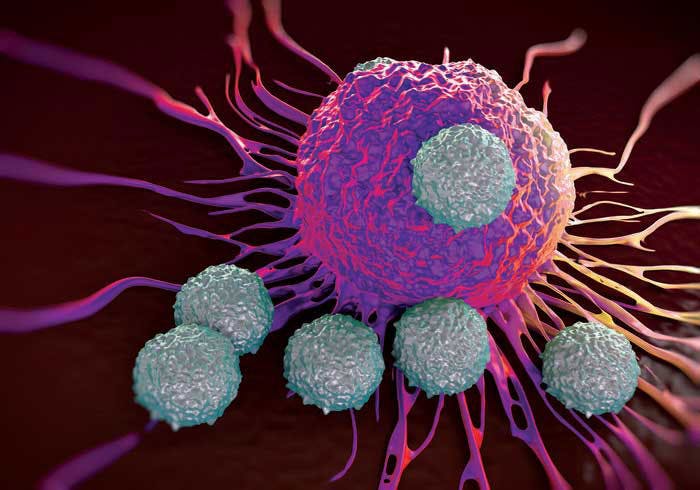
In the past decade, immunotherapies have revolutionized the way we treat cancer. By activating the immune system, these powerful treatments are able to use the body's own defense mechanisms to attack cancer cells and halt tumor growth.
Scientists at the University of Chicago Medicine Comprehensive Cancer Center are leading the field of immunology, from basic research that aims to understand how the immune system works at the molecular level to clinical trials that are testing the newest and most promising therapies.
However, immunotherapy is not always effective. No two cancers are alike, and genetic, biological and environmental factors can make some people less likely to respond to immunotherapy. So, how do you overcome this barrier and develop better therapies that can help more patients?
Understanding patient response to immunotherapy
Our researchers have uncovered several factors that play a role in a patient's response to immunotherapy. For example, Thomas Gajewski, MD, PhD, professor of pathology and medicine, recently found that a person's gut bacteria, or microbiota, may impact the effectiveness of certain immunotherapies (learn more).
Another key player is the tumor microenvironment the cellular environment in which a tumor lives. The microenvironment consists of tissue, blood vessels, immune cells, fibroblasts, and other components that interact with the tumor cells. As it turns out, the tumor's "home" plays an important role in how tumors behave and their susceptibility to certain drugs.
Gajewski coined the term "T-cell-inflamed tumor microenvironment" to describe a major subset of tumors from patients whose cellular environments harbor qualities that make them more likely to respond to immunotherapies.
More than two decades ago, researchers began testing cancer vaccines that aimed to elevate a person's level of antigen-specific T cells white blood cells that can recognize antigens, or short protein fragments, displayed on the surface of cancer cells. The scientists observed that some people already had elevated levels of T cells in their blood prior to vaccination, and the vaccine was ineffective at sparking a response in others. Gajewski's team set out to decode this dilemma more than a decade ago.
Gajewski and his team analyzed tumor tissue samples from patients with metastatic melanoma and found that certain tumor microenvironments contain cytotoxic (CD8+) T cells, a type of white blood cell that can recognize and kill cancer cells. They also identified the presence of inoleamine-2, 3-dioxygenase (IDO), programmed death-ligand 1 (PD-L1), and Foxp3+ regulatory T (Treg) cells, which are all inhibitory mechanisms that can restrict the function of tumor-specific CD8+ T cells
.
The IDO enzyme, PD-L1 molecule, and Foxp3+ Treg cells all play a role in regulating the immune system by keeping T cells in check (learn more). Thus, Gajewski and his teamed originally hypothesized that the presence of these immune-suppressing mechanisms would make patients less responsive to vaccine therapies.
However, the exact opposite turned out to be true. The patients with tumors containing these T-cell-inflamed tumor microenvironments had a better clinical response. Because IDO, PD-L1 and Foxp3+ are associated with the presence of CD8+ T cells in the tumor microenvironment, their presence actually predicts response to vaccines.
"More importantly, this suggested that IDO, PD-L1, and Tregs might be targets for new therapy development-eliminating these inhibitory pathways could be therapeutic," Gajewski said.
Pioneering new treatments
Checkpoint inhibitor immunotherapies help the immune system mount an attack by deactivating specific proteins or molecules that control immune checkpoints, which cancer cells use to avoid detection.
PD-L1 is one such checkpoint target, along with programmed cell death protein 1 (PD-1), which is the protein PD-L1 binds to. PD-L1 and PD-1 are found on the surface of both normal and cancer cells, and help prevent the immune system from running rampant by limiting T-cell overactivation. However, this can hinder the body's ability to fight cancer. Inhibitor drugs "turn off" these otherwise well-meaning proteins and molecules so the body can mount a robust immune response.
Multiple clinical trials at the University of Chicago Medicine are testing combination therapies of PD-1 or PD-L1 inhibitors with other checkpoint inhibitors in various tumor types, including melanoma, bladder cancer, ovarian cancer, and triple-negative breast cancer, to name a few.
In addition, researchers have found that combining immunotherapies with other treatments may provoke an even better clinical response in patients than either treatment alone.

Ralph Weichselbaum, MD, Daniel K. Ludwig Distinguished Service Professor of Radiation and Cellular Oncology, discovered that radiation therapy can improve the efficacy of immunotherapies.
Radiation helps activate CD8+ T cells in tumors, while a checkpoint inhibitor helps protect those T cells from immune suppression. In one study, Weichselbaum added a vaccine to the mix and found that the combination of the three therapies helped overcome the non-T-cell-inflamed tumor microenvironment in pancreatic cancer.
"Our results provide a step-by-step strategy to break the immune barriers that protect aggressive tumors by converting so-called 'cold,' or non-T-cell-inflamed, tumors to a 'hot,' or T-cell-inflamed, phenotype," said Weichselbaum.
A phase I clinical trial led by Jason Luke, MD, assistant professor of medicine, and including Steven Chmura, MD, PhD, associate professor of radiation and cellular oncology, combines the PD-1 inhibitor pembrolizumab with stereotactic body radiotherapy (SBRT) in patients with advanced solid tumors. SBRT is a type of radiation therapy that delivers precise doses of radiation to tumors in the body while limiting damage to normal tissue.
SBRT will also be combined with immunotherapy in upcoming clinical trials for liver cancer, led by Manish Sharma, MD, assistant professor of medicine.
Other researchers leading immunotherapy clinical trials include:
- Rita Nanda, MD, assistant professor of medicine, in triple-negative breast cancer
- Hongtao Liu, MD, PhD, assistant professor of medicine, in hematologic malignancies
- Tanguy Seiwert, MD, assistant professor of medicine, in head and neck cancer
- Hedy Kindler, MD, professor of medicine, in mesothelioma
- Peter O'Donnell, MD, assistant professor of medicine, in bladder cancer
- Everett Vokes, MD, John E. Ultmann Professor of Medicine and Radiation Oncology, in non-small cell lung cancer
Making "cold" tumors "hot"
Another protein that plays an important role in innate immunity the body's natural ability to mount a defense is the Stimulator of Interferon Genes (STING) protein. In 2014, Gajewski and colleagues found that the STING pathway triggers a natural immune response against tumor cells and helps jump start the production of CD8+ T cells.
This observation generated the idea that pharmacologic stimulation of STING could prove useful for patients with non-T-cell-inflamed tumor microenvironments, or "cold" tumors, who normally don't have a robust response to immunotherapy. This proved to be the case, and STING agonists (chemicals that stimulate a biological response) were found to have profound therapeutic activity in mouse models of cancer.
Justin Kline, MD, assistant professor of medicine, and Emily Curran, MD, clinical instructor of medicine, are studying the STING pathway in acute myeloid leukemia (AML). Using mouse models, they were able to stimulate STING by injecting substances that mimic tumor-cell DNA into the bloodstream.
"Delivery of these substances into the blood led to massive immune responses," said Kline. "I've worked extensively with animal models of this disease, and have never seen responses like this."
Based on this work and a licensing arrangement with Aduro Biotech, University of Chicago researchers are conducting clinical trials testing the first STING agonist in melanoma, head and neck cancer, and triple-negative breast cancer.
The future of immunotherapy
New and promising types of immunotherapy have emerged in the past five years. For example, CAR T-cell therapy for acute lymphoblastic leukemia (ALL) and other blood cancers involves engineering a person's own immune cells to recognize and destroy cancer cells.For patients suffering from relapsed/refractory ALL or other B-cell malignancies, CAR T-cell therapies may give doctors who have exhausted traditional chemotherapies another tool to use.
Michael Bishop, MD, professor of medicine, is leading CAR-T cell trials at the University of Chicago and has seen promising patient outcomes (learn more).
Collaborative and transdisciplinary research efforts are helping elevate immunotherapies to a new level.
Wenbin Lin, PhD, James Franck Professor of Chemistry, and colleagues including Weichselbaum are making checkpoint inhibitor immunotherapies more effective through the use of photodynamic therapy (PDT), a treatment that uses a drug called a photosensitizer. When photosensitizers are exposed to a specific wavelength of light, they produce a lethal type of oxygen that can kill cancer cells.
Gajewski and Luke will lead a phase II study that examines how changing a person's gut bacteria can impact the efficacy of pembrolizumab in melanoma. Still in early stages, the trial will be supported by Evelo Biosciences, a leading immune-microbiome company.
In addition, basic science researchers are working to understand the basic wiring of the immune system, laying the groundwork for the development of future cancer therapies. Investigators like Hans Schreiber, MD, PhD, professor of pathology, and Peter Savage, PhD, associate professor of pathology, are leading their fields in cancer immunology.
These are just a few examples of how our researchers' innovative and targeted approaches are shaping the future of immunotherapy, ensuring more effective treatments and better patient outcomes.
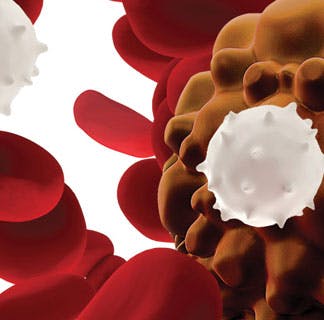
CAR T-Cell Therapy
CAR T-cell therapy supercharges a patient's white blood cells to find and destroy cancer cells. UChicago Medicine research played a key role in the development of this exciting new immunotherapy.
View videos and learn more
Participate in a Cancer Clinical Trial
UChicago Medicine cancer experts are actively conducting clinical trials of new screening methods and treatments for cancer, including breakthrough approaches to harnessing the immune system to fight cancer. As a recognized leader in phase I and other early-phase clinical trials, our physician-scientists are creating new cancer treatment methods that later become the standard of care elsewhere.
Find a cancer clinical trial